Thursday, July 7, 2011
2SAS #26-34, p. 132 and 2SBS #1-8, p.151
2SAS:
26) Which are more likely to lose electrons, metallic elements or nonmetallic elements?
Metallic elements form cations; therefore, metallic elements are more likely to lose electrons.
27) Noble gas elements rarely lose or gain electrons. What does this indicate about their chemical reactivity?
Noble gas elements are unreactive and chemically inert.
28) Predict whether each of the following elements would be more likely to form an anion or a cation: (Note: Anions are negatively charged; cations are positively charged.)
a. Na
b. Ca
c. F
d. Cu
e. O
f. Li
g. Sn
h. I
a. cation.
b. cation.
c. anion.
d. cation.
e. anion.
f. cation
g. cation.
h. anion.
29) Which pair is more similar chemically? Defend your choice:
a. copper metal and copper (II) ions
or
b. oxygen with mass number 16 and oxygen with mass number 18
a. copper metal and copper (II) ions: copper metals form cations, and the only difference between copper metal and copper (II) ions is the absence of 2 electrons. Oxygen with mass number 18 as opposed to 16 exhibits an isotope. Neutrons are way bigger than electrons which equal 1/2000 neutrons, so a change in neutrons is a bigger change.
30) The diameter of a magnesium ion (mg^2+) is 156 pm (picometers, where 1 pm = 10^-12 m); the diameter of a strontium ion (Sr^2+) is 254 pm. Estimate the diameter of a calcium ion (Ca^2+).
156+254=410
410/2=205; 205 pm.
The diameter of a calcium ion (Ca^2+) = 205 pm.
31) Three kinds of observations that may indicate a chemical change appear in the following list. However, a physical change may also result in each observation. Describe a possible chemical cause and a possible physical cause for each observation:
a. change in color.
b. change in temperature.
c. formation of a gas.
a.
Chemical cause: oxidization or rusting results in a change in color.
Physical cause: painting a substance results in a change in color.
b.
Chemical cause: combustion causes a change in temperature.
Physical cause: boiling a substance will raise the temperature.
c.
Chemical cause: the reaction of HCl and Cu causes the formation of a gas.
Physical cause: after boiling, water will become a gas (water vapor) as it evaporates.
32) Identify the element that is described by each of the following statements:
a. This element is a nonmetal. It forms anions with a 1- charge. It is in the same period as the metals used in a penny.
b. This element is a metalloid. It is in the same period as the elements found in table salt.
a. Bromine (Br).
b. Silicon (Si).
33) Compare your use of the Snake River data to solve the fish-kill mystery in Unit 1 to Mendeleev's use of element data to create the periodic table.
Mendeleev used atomic weight along with similarities in chemical and physical properties to organize the periodic table. In analyzing our Snake River data to solve the fish-kill mystery in Unit 1, we had to compare mass changes among the different substances we tested for with the normal masses. We used our knowledge of properties of the different substances to create legitimate and logical hypotheses.
34) Mendeleev arranged elements in his periodic table in order of their atomic weights. In the modern periodic table, however, elements are arranged in order of their atomic numbers. Cite two examples from the periodic table for which these two schemes would produce a different ordering of adjacent elements.
Argon (atomic weight: 39.95) would have had to be placed after potassium (atomic weight: 39.10), and cobalt (atomic weight: 58.93) would have had to be placed after nickel (58.69) in order to be accurate in Mendeleev's original periodic table.
2SBS:
1) List two resources typically found in each of the three major "spheres" of Earth.
Atmosphere: nitrogen, oxygen, neon, and argon.
Hydrosphere: water and dissolved minerals.
Lithosphere: petroleum and metal-bearing ores.
2)
a. List and briefly describe three major parts of the lithosphere.
b. Which layer serves as the main storehouse of chemical resources used in manufacturing consumer products?
a.
The crust: top 40km: the thin band of soil and rock containing major raw materials needed to build all manufactured objects.
The mantle: 40-2900km: the middle layer of the lithosphere.
The core: 2900km: Earth's center: extremely hot.
b.
The crust serves as the main storehouse of chemical resources used in manufacturing consumer products.
3) Identify the nation that produces the most
a. silver.
b. copper.
c. tin.
a. Mexico.
b. Japan.
c. China.
4) According to the information in Table 2.3 on page 136, which of these four nations-- the US, Australia, China, or Brazil-- produces the largest masses of the eight listed resources in the table?
China produces the largest masses of the eight listed resources in the table.
5) How do minerals differ from ores?
Ores are naturally occurring rocks or minerals that can be mined and from which are profitable to extract a metal or other metal. Minerals are naturally occurring solid compounds containing the element or group of elements of interest.
6) What factors determine the feasibility of mining a particular metallic ore at a certain cite?
-the quantity of useful ore found at the site.
-the percent of metal in the ore.
-the type of mining and processing needed to extract the metal from its ore.
-the distance between the mine and metal-refining facilities and markets.
-the metal's supply-versus-demand status.
-the environmental impact of the mining and metal processing.
7) A nineteenth-century gold mine, inactive for over 100 years, has recently reopened for further mining. What factors may have influenced the decision to reopen the mine?
Maybe the gold had replenished after 100 years and demand for gold was high.
8) What is meant by referring to the quantity of "useful ore" at a sight.
Useful ore refers to the amount, usually as a percent, of the desired mineral being mined.
Extra Credit for Friday, July 8th: Mental well-being: A New York state: Urban brains behave differently from rural ones of mind:
Mental well-being
A New York state of mind
Urban brains behave differently from rural ones
Jun 23rd 2011 | from the print edition
“HELL is a city much like London,” Percy Bysshe Shelley in 1819.
Building off of Dutch researchers' discovery that city dwellers have a 21% higher risk of developing anxiety disorders and a 39% higher risk of developing mood disorders, Andreas Meyer-Lindenberg of the University of Heidelberg and his colleges used a scanning technique, functional magnetic-resonance imaging (fMRI) to examine the brains of city dwellers and those who live in rural areas under stressful conditions. Dr. Meyer-Lindenberg conducted several experiments; in the first experiment, he had both urban and rural participants of the same general mental healths lay down with their heads in a scanner. The participants were monitored for indications of stress, like high blood pressure, as they took impossible math tests designed for failure while receiving negative feedback through headphones. The brains of the urbanites and country dwellers reacted to the stress very differently in the amygdalas, a pair of structures divided among the two cerebral hemispheres, located deep inside the brain, and responsible for assessing threats and generating fearful emotions, and the perigenual anterior cingulate cortex (pACC), that regulates the amygdalas. In the case of the amygdalas, those who lived in the countryside had the lowest levels of activity in their amygdalas, where as the city dwellers had the highest levels of activity in their amygdalas. Because of the pACC's role in regulating the amygdalas, changes of the pACC may alter the amygdalas, however, the activity and reactions of the pACC are not flexible like the activity of the amygdalas, but are determined during childhood. In other words, a more urban childhood results in a more active pACC, but the activity of the amygdalas reflects where one is currently dwelling. The fMRI measured these correlations between the amygdalas and the pACC. The fMRI reflected the expectations of Dr. Meyer-Lindenberg and his team. The activity of the pACC for the native urbanite showed to be out of kilter. Furthermore, it has been proven that schizophrenia is more common among urbanites than rural dwellers, and that the pACC-amygdala link is quite often out of kilter in schizophrenia; however, Dr. Meyer-Lindenberg is resistent in claiming that his test results show the cause of the out-of-kilter connection. In order to check their results, Dr. Meyer-Lindenberg and his team conducted several subsequent experiments and assigned participants additional stress-free tasks; the subsequent tests matched the results of the original test conducted, and the results of the stress-free tasks showed that the first test was most definitely of social stress, not mental exertion. Therefore, urban brains indeed behave differently than rural ones under stressful situations.
http://www.economist.com/node/18864354
A New York state of mind
Urban brains behave differently from rural ones
Jun 23rd 2011 | from the print edition
“HELL is a city much like London,” Percy Bysshe Shelley in 1819.
Building off of Dutch researchers' discovery that city dwellers have a 21% higher risk of developing anxiety disorders and a 39% higher risk of developing mood disorders, Andreas Meyer-Lindenberg of the University of Heidelberg and his colleges used a scanning technique, functional magnetic-resonance imaging (fMRI) to examine the brains of city dwellers and those who live in rural areas under stressful conditions. Dr. Meyer-Lindenberg conducted several experiments; in the first experiment, he had both urban and rural participants of the same general mental healths lay down with their heads in a scanner. The participants were monitored for indications of stress, like high blood pressure, as they took impossible math tests designed for failure while receiving negative feedback through headphones. The brains of the urbanites and country dwellers reacted to the stress very differently in the amygdalas, a pair of structures divided among the two cerebral hemispheres, located deep inside the brain, and responsible for assessing threats and generating fearful emotions, and the perigenual anterior cingulate cortex (pACC), that regulates the amygdalas. In the case of the amygdalas, those who lived in the countryside had the lowest levels of activity in their amygdalas, where as the city dwellers had the highest levels of activity in their amygdalas. Because of the pACC's role in regulating the amygdalas, changes of the pACC may alter the amygdalas, however, the activity and reactions of the pACC are not flexible like the activity of the amygdalas, but are determined during childhood. In other words, a more urban childhood results in a more active pACC, but the activity of the amygdalas reflects where one is currently dwelling. The fMRI measured these correlations between the amygdalas and the pACC. The fMRI reflected the expectations of Dr. Meyer-Lindenberg and his team. The activity of the pACC for the native urbanite showed to be out of kilter. Furthermore, it has been proven that schizophrenia is more common among urbanites than rural dwellers, and that the pACC-amygdala link is quite often out of kilter in schizophrenia; however, Dr. Meyer-Lindenberg is resistent in claiming that his test results show the cause of the out-of-kilter connection. In order to check their results, Dr. Meyer-Lindenberg and his team conducted several subsequent experiments and assigned participants additional stress-free tasks; the subsequent tests matched the results of the original test conducted, and the results of the stress-free tasks showed that the first test was most definitely of social stress, not mental exertion. Therefore, urban brains indeed behave differently than rural ones under stressful situations.
http://www.economist.com/node/18864354
The Acids: Converting Copper Lab
Notes: Procedure:
• Copper powder before the heating is a fine brick-red powder substance
• After the two minutes being on the hot plate, the substance was charcoal and stiff with a slightly purple hue
• Then it was removed from the hot plate and broken up as much as possible with a spatula into a not as fine powder
- Then we placed the crucible back on the hot plate for 10 more minutes with the top ajar to let some oxygen in
• Every two minutes we removed the crucible from the hot plate and broke up the solid with a spatula as previously done
• After the first 2 minutes, it remained a powdery substance with more solid particles
- Instead of having a purplish hue it had a charcoal color
• After the next two minutes the substance remained the same
• After the next two minutes the substance remained the same
• Then we removed the crucible from the hot plate, placed it on the base of the ring-stand, and allowed its contents to cool to room temperature
Questions: Page 140:
1. Answers
a. Describe changes you observed as you heated the copper.
i. We observed color change and the formation of solid particles within the heated crucible. In the first stages of heating, the copper became charcoal in color with a slightly purple hue, and by the end of the heating cycle, the copper was completely charcoal in color.
b. Did the copper atoms remain in the crucible? Explain, using evidence from your observations.
i. Yes the copper did remain inside the crucible but there were slight powder stains on the sides of the crucible that changed color along with the rest of the powder.
2. Answers
a. Were the changes you observed physical changes or chemical changes?
i. As we observed, the copper oxidized; therefore, the changes were chemical changes.
b. What observational evidence leads you to that conclusion?
i. It is a chemical reaction because burning, or combustion, involves chemical reactions between the copper and the oxygen.
3. Answers
a. How did the mass of the crucible contents change after you heated the copper?
i. The mass of the contents did not change at all, they were exactly the same before and after heating
b. Explain why the mass of the crucible contents change in that manner.
i. Although we recognized that the mass of the crucible content should have increased after oxidization, it remained the same. This may be due to crushing the powder too fine, or because of not enough copper particles oxidizing.
Aggregated Data:
Average gain: 0.06g
Median gain: 0.06g
Wednesday, July 6, 2011
2SAS #13-25, p. 131-132
13) Give another term for each of these features of the periodic table:
a. row.
b. column.
a. A horizontal row is also a period, with elements listed in order of increasing atomic numbers.
b. A vertical column is a group, that contains elements with similar properties.
14) Give the names and symbols of two elements other than lithium in the alkali metal family.
Sodium (Na) and Potassium (K).
15) Consider the noble gas family:
a. Where are noble gases located on the periodic table?
b. Name one physical property that noble gases share.
c. Name one chemical property that noble gases share.
a. The noble gases are located on the far right side of the periodic table.
b. Noble gases are unreactive.
c. Noble gases are chemically inert.
16) Given a periodic table and the formulas BeCl2 and AlN, predict the formula for a compound containing
a. Mg and F.
b. Ga and P.
a. MgF2
b. GaP
17) The melting points of sodium (Na) and rubidium (Rb) are 98°C and 39°C respectively. Estimate the melting point of potassium (K).
Potassium is right between sodium and rubidium on the periodic table:
98+39=137
137/2=68.5
Melting point: 68.5°C.
18) Would you expect the boiling point of chlorine to be higher or lower than that of iodine? Explain.
I would expect the boiling point of chlorine to be lower than that of iodine. From what I have observed, I believe that elements with higher atomic weights have higher boiling points than those with lower atomic weights. Chlorine has a lower atomic weight (35.45) than iodine (126.90), so, chlorine must have a lower boiling point.
19) Copy and complete the following table for each electrically neutral atom.
Completed chart:
20) Using Figure 2.11 (page 121) as a model, illustrate the number of protons, neutrons, and electrons in an atom of
a. beryllium.
b. nitrogen.
c. neon.
a. 4 protons, 4 or 5 neutrons, 4 electrons.
b. 7 protons, 7 or 8 neutrons, 7 electrons.
c. 10 protons, 10 or 11 neutrons, 10 electrons.
21) A student is asked to explain the formation of a lead (II) ion (Pb^2+) from an electrically neutral lead atom (Pb). The student says the a lead atom must have gained two protons to make the ion. How would you correct this student's mistaken explanation?
Although protons are positive, when an atom forms an ion, it either loses or gains electrons. Since a lead ion with a 2+ charge was formed, the atom must have lost two electrons; when electrons are lost, there are more protons remaining in the atom, resulting in the formation of cations, positively charged ions.
22) Refer to the table provided for Question 19:
a. Calculate the mass number for each element in the table.
b. Which element has two isotopes in the table?
a.
Carbon: 6+6=12, or 6+7=13; 12 or 13
Calcium: 20+21=41; 41
Platinum: 78+117=195; 195
Uranium: 92+146=238; 238
b. Carbon has two isotopes in the table.
23) A scientist announces the discovery of a new element. The only characteristic given in the report is the element's mass number of 266. Is this information sufficient, by itself, to justify the claim of the discovery of a new element? Explain.
No, the discovery of a new element would not be justified by only its mass number. It would be more helpful to know either the atomic weight or atomic number in order to be able estimate physical and chemical properties of the element and its probable spot on the periodic table. An element's mass number does not provide enough information to justify its existence.
24) How does the mass of an electron compare to the masses of a proton and a neutron?
The mass of an electron is about 1/2000 the mass of a proton or a neutron. Because of its minuscule weight, it is insignificant, and not used to calculate the mass number of an atom.
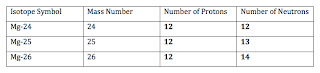
25) How many protons and neutrons are needed for each magnesium isotope in this table?
Completed Chart:
Periodic Table and Graphs: Trends in a Chemical Property and Trends in a Physical Property
Questions, Page 123:
1) Does either bar graph reveal a repeating, or cyclic, pattern? Describe any patterns you observe.
By looking at both bar graphs side by side, we noticed that the number of atoms added to the element directly correlated to the boiling points (in °K). The more atoms added, the higher the boiling point.
2) Are these graphs consistent with patterns found in your earlier grouping of the elements? Explain.
Yes. We grouped our elements based on the atom's charge. Elements with positively charged ions were generally on the right side of the table-- closer to what we classified as noble gases. Elements with negatively charged ions were put on the left side of the table, with one positively charged ion set away from the rest of the table on the top. Although the modern periodic table organizes those elements that become cations on the left side of the table, we put the elements that became the cations on the right side of the table, closer to the noble gases, due to the closer atomic numbers and element names (or numbers). Even so, our version of the periodic table sort of imitates the modern periodic table. Additionally, we noticed that positively charged ions generally had higher boiling points than those with negative charges.
3) Based on these two bar graphs, why is the chemist's organization of elements called a periodic table?
Based on these two bar graphs, the chemist's organization of elements is called a periodic table because it is organized in a logical and methodical way, creating patterns that are fairly simple to understand by simply looking at the table.
4) Where are elements with the highest oxide numbers located on the periodic table?
On our periodic table, the elements with the highest oxide numbers are located on the far right and far left of the table (the right side being for the most positively charged ions, and the left side being for the most negatively charged ions).
5) Where are elements with the highest boiling points located on the periodic table?
On our periodic table, the elements with the highest boiling points are located toward the right side of the table. We noticed that the more positive charges the ion had, the higher the boiling point for the element was.
6) Explain any trends you noted in your answers to Question 4 and 5.
The elements with the highest oxide numbers tended to have the highest boiling points.
7) Predict which element should have the lowest boiling point: selenium(Se), bromine(Br), or krypton(Kr). Use evidence from your graphs to explain how you decided.
Bromine should have the lowest boiling point. Out of the three elements, this element is closest to the noble gas (which does not gain any electrons to become an ion), krypton. Bromine has a positive one charge when it is an ion, and selenium has a positive two charge when it is an ion. By analyzing our graph, we noticed that generally, the higher the positive charge, the higher the boiling point of the element; therefore, bromine should have the lowest boiling.
8) Using your graphs, predict the pattern in boiling points and oxide numbers for the next 5 to 8 elements, starting with gallium.
Using our graphs, we believe the higher the oxide numbers, the higher the boiling points will be.
Tuesday, July 5, 2011
2SAS #1-12, p. 130
1) Classify each of the following as a chemical or a physical property:
a. copper has a reddish brown color.
b. propane burns readily.
c. CO2 gas extinguishes a candle flame.
d. Honey pours more slowly than does water.
a. Physical property.
b. Chemical property.
c. Chemical property.
d. Physical property.
2) Classify each of the following as a chemical or a physical property:
a. Metal wire can be bent.
b. Ice floats in water.
c. Paper is flammable.
d. Sugar is soluble in water.
a. Physical property.
b. Physical property.
c. Physical property.
d. Chemical property.
3) Classify each of the following as a chemical or a physical change:
a. a candle burns.
b. an opened carbonated beverage fizzes.
c. hair curls as a result of a "perm."
d. as shoes wear out, holes appear in the soles.
a. Chemical change.
b. Physical change.
c. Chemical change.
d. Physical change.
4) Classify each of the following as a chemical or a physical change:
a. a cut apple left out in the air turns brown.
b. flashlight batteries lose their "charge" after extended use.
c. dry cleaning removes oils from clothing.
d. Italian salad dressing separates over time.
a. Chemical change.
b. Chemical change.
c. Physical change.
d. Physical change.
5) For each of your answers in Question 4, give evidence for your classification as a chemical or physical change.
a. The atmospheric gases other gases react with the inner part of the apple and causes it's top layer to decay.
b. The flashlight batteries contain chemicals, and once stored energy is gone, the chemical reaction is reversed to get electricity out of the battery.
c. The oils in the clothing are simply removed from the clothing, but the clothing remains the same.
d. The substances within mixture of salad dressing gradually separate and suspend within the container, a chemical change does not occur.
6)
a. List the steps involved in making chocolate chip cookies from scratch.
b. Classify each step in Question 6a as involving either a chemical change or a physical change.
a. Mix ingredients such as milk, eggs, sugar, flour, and chocolate chips together in a bowl. Then, put the batter on a baking sheet and place it in the oven. When taken out of the oven, allow to cool.
b. A chemical change occurs when all the ingredients are mixed together and heated in the oven. they form cookies, one substance formed after all the ingredients are mixed together and heated. Coming out of the oven and cooling is a physical change, the heat of the cookie changes, not the cookie itself.
7) Classify each property as characteristic of metals or nonmetals:
a. shiny in appearance.
b. does not react with acids.
c. shatters easily.
d. electrically conductive.
a. metals.
b. nonmetals.
c. nonmetals.
d. metals.
8) Classify each of these elements as a metal, a nonmetal, or a metalloid:
a. tungsten
b. antimony
c. krypton
d. sodium
a. metal.
b. metalloid.
c. nonmetal.
d. metal.
9) List the names and symbols of two elements that are metalloids.
1) Silicon: Si
2) Arsenic: As
10) What would you expect to happen if you tapped a sample of each of the following elements with a hammer?
a. iodine.
b. zirconium.
c. phosphorus.
d. nickel.
a. it would shatter (brittle).
b. it would flatten (malleable).
c. it would shatter (brittle.
d. it would flatten (malleable).
11) List two properties that make nonmetals unsuitable for electric wiring.
Nonmetals do not conduct electricity and are not malleable; therefore, nonmetal wiring would be brittle and unable to bend very well and would not even be able transmit electricity!
12) List three properties that make metals suitable for use in coins.
1) Metals are malleable, therefore, they can be engraved with currency symbols and political leaders.
2) Metals are shiny in color and make more attractive coins!
3) Metals aren't easily shattered or broken, for they aren't brittle like nonmetals.
Lab Report: Metal or Nonmetal Lab
Abstract:
For this lab experiment, our new group, The Acids, tested sample elements A-G with the hopes in gaining enough knowledge to characterize each sample as a metal, a nonmetal, or a metalloid. To prepare for the simple, yet tedious procedure, we placed each of the seven elements on a piece of paper, and labeled them to prevent confusion. Then, after recording the initial appearances of each sample on our data table, we were ready to begin the procedure that required plenty of focus and copious notes. The process included observing appearance, conductivity, crushing, reactivity with copper (II) chloride, and reactivity with hydrochloric acid. We entered the experiment with the knowledge that metals have a luster, are malleable, and conduct electricity, nonmetals are usually dull, brittle, and do not conduct electricity, and metalloids have some properties of both. After crushing each sample on our piece of paper, and then labeling and filling our wellplate with each sample twice, Rachel used the electrical conductivity apparatus to test the conductivity of each sample. Then, we added 20 drops of copper (II) chloride to the left well of each set, and 20 drops of hydrochloric acid to the right well of each set. After about three minutes, we analyzed whether or not there was a reaction, and what kind of reaction occurred in each wellplate. Then, we recorded the data in our data table. Although it was difficult deciphering those elements that possessed contradictory physical and chemical properties, we analyzed our detailed data table and, with our knowledge of the three categories, came to the conclusion that sample elements B and G were metals, sample elements A and E were nonmetals, and sample elements C, D, and F were metalloids.
Procedure:
Even before beginning the procedure, our group, The Acids, spent more time than other groups to organize our notes, data table, element samples, and labels with the motive of conducting a quick, smooth, and accurate experiment. First, we gathered all the tools needed to begin the experiment: two plastic wellplates, a notebook, a pen, a piece of paper, scotch tape for labeling, a hammer-like object, copper (II) chloride, and hydrochloric acid. Then, we waited as Rachel distributed sample elements A-G to each lab group. Before crushing the elements, we waited until all samples were placed on our piece of paper and labeled with their corresponding letters.
We also ripped pieces of scotch tape in half, and labeled two of the same letter on each piece; we attached these pieces of tape to the wellplates, assigning the same letter to each set of two wells. After we were sure we had everything organized, we documented the initial appearances of each sample onto our data table very carefully and in great detail to ensure accurate results. From the data of the appearances alone, sample A appeared to be nonmetallic, samples B and C appeared to be metallic, and samples D, E, F, and G were undecided. Once we were sure everything was organized, we were ready to crush each sample element to decide whether they were malleable or brittle.
Since sample A was originally a powder and remained a powder, samples C , E, and F were brittle, and sample D was very hard to break and sort of brittle, we recorded them as nonmetallic. Because sample B was clearly malleable, we recorded it as a metal. Due to brittleness, yet shiny and hollow chambers revealed in sample G, we recorded it as undecided. After crushing, we divided each sample element in half and placed them side-by-side in the wells in the labeled wellplate.
Rachel tested each crushed sample element’s electrical conductivity with the electrical conductivity apparatus; even if the light bulb was only slightly lit, the light indicated electrical conductivity.
Sample elements A and E were not conductive and sample elements B, C, D, F, and G were conductive—metals conduct electricity. Next, we were ready to fill the wells with copper (II) chloride and hydrochloric acid. We decided to fill the left well of each set with copper (II) chloride, and the right side of each set with hydrochloric acid. After placing 20 drops of each into the wells, we had to wait for a reaction (if any) for three to five minutes.
After three minutes, we analyzed each well set. On the copper chloride side of each sample, the left side of each set, we observed that the liquid simply sat in a pool around each sample for all samples except for sample G. In copper (II) chloride well G, the sample turned black, and a black substance began to come off of the sample. With this data, we recorded only sample G as metallic. On the hydrochloric acid side of each sample, the left right side of each set, again, the liquid sat in a pool around each sample. This time, there were two exceptions in hydrochloric acid well D and, again, well G. The reaction in well D was not very prominent, but we noticed that some bubbles formed in the clear liquid around our sample. In well G, we noticed many bubbles coming off and popping off of our sample that appeared to be dissolving. From this data, we concluded that samples D and G were metallic.
After making sure we had all of our notes down and data table complete, we cleaned up our supplies and went on to analyze the properties of each sample element to reach a conclusion as to what was metallic, nonmetallic, or a metalloid.
Results:
Although this lab may seem simple, our group had some trouble classifying our cumulative data into metal, nonmetal, or metalloid. The metals and nonmetals were very clearly shown in our results from the five observations we produced, but due to contradicting properties, the metalloids were not. Just looking at the initial appearance of our samples to decipher if each sample was a metal or nonmetal was complicated because many samples such as D, E, F, and G contained small particles of shiny bits, or tiny specs of luster. Drawing conclusions from just appearance would often lead to inaccurate data, so we had to use our conclusions from the tests for electrical conductivity, crushing, and chemical reactions with copper (II) chloride and hydrochloric acid as well. Since a lot of the data per sample was contradictory, it made it difficult to decide what category each fit into. In addition, the concept of deciding how a sample could be a metalloid was very unfamiliar to us, so we had to review in our book that although metalloids have properties intermediate to metals and nonmetals, sometimes the absence of a property that defines a metal or nonmetal does not alienate it from being part of one of those categories. After difficult analysis, we concluded that element samples C, D, and F were metalloids, element samples A and E were nonmetals, and element samples B and G were metals.
Data Analysis:
1.Classify each property tested in this investigation as either a physical property or a chemical property.
Appearance: Physical property
Conductivity: Physical property
Crushing: Physical property
Reactivity with copper (II) chloride: Chemical property
Reactivity with acid: Chemical property
2. Sort the seven coded elements into two groups based on similarities in their physical and chemical properties.
1st Group: (Metals) B, G
2nd Group: (Non-Metals) A, C, D, E, F
3. Which element or elements could fit into either group? Why?
Elements C, D, and F could fit into either group—metalloids. These elements could be metalloids because although some of them did or did not show to posses conductivity, some were brittle and did not react with either acid solution, and some did. Because these elements were contradictory in their cumulative results for the tests, they could be classified as metalloids.
4. Using the following information, classify each tested element as a metal, a nonmetal, or a metalloid:
-Metals have a luster, are malleable (can be hammered into sheets), and conduct electricity.
-Many metals react with acids; many metals also react with copper (II) chloride solution.
-Nonmetals are usually dull in appearance, are brittle, and do not conduct electricity.
-Metalloids have some properties of both metals and nonmetals
Metals: Elements B and G.
Nonmetals: Elements A and E.
Metalloids: Elements C, D, and F.
Data Table:
Conclusion:
After having a class discussion, we discovered that we were accurate in almost all of our conclusions based on our data, with two exceptions. We discovered that element A, that we classified as a nonmetal, was aluminum (Al), a metal. This mistake occurred because aluminum is right on the edge of the break between metals and nonmetals, above some metalloids. Because of its closeness to the nonmetals on the periodic table, our mistake in confusing aluminum, a metal, for a nonmetal, was not uncommon. Additionally, the aluminum sample was in powder form, and powder rarely conducts electricity. Our second mistake was with element sample F, Carbon (C). On the periodic table, carbon is a nonmetal, but we classified it as a metalloid. Because carbon conducted electricity but was brittle and did not react with either copper (II) chloride and hydrochloric acid, we thought that since metals conduct electricity, it would be a metalloid. Now we realize that even though carbon conducts electricity, it is a nonmetal. Despite these two errors, we were correct in our conclusions for sample elements B, C, D, E, and G: element B was tin (Sn), a metal, element C was Silicon (Si), a metalloid, element D was Antimony (Sb), a metalloid, and element G was zinc (Zn), a metal. Overall, this lab experiment was very helpful in understanding the differences between metals, nonmetals, and metalloids, and how to characterize elements based on experiments.
For this lab experiment, our new group, The Acids, tested sample elements A-G with the hopes in gaining enough knowledge to characterize each sample as a metal, a nonmetal, or a metalloid. To prepare for the simple, yet tedious procedure, we placed each of the seven elements on a piece of paper, and labeled them to prevent confusion. Then, after recording the initial appearances of each sample on our data table, we were ready to begin the procedure that required plenty of focus and copious notes. The process included observing appearance, conductivity, crushing, reactivity with copper (II) chloride, and reactivity with hydrochloric acid. We entered the experiment with the knowledge that metals have a luster, are malleable, and conduct electricity, nonmetals are usually dull, brittle, and do not conduct electricity, and metalloids have some properties of both. After crushing each sample on our piece of paper, and then labeling and filling our wellplate with each sample twice, Rachel used the electrical conductivity apparatus to test the conductivity of each sample. Then, we added 20 drops of copper (II) chloride to the left well of each set, and 20 drops of hydrochloric acid to the right well of each set. After about three minutes, we analyzed whether or not there was a reaction, and what kind of reaction occurred in each wellplate. Then, we recorded the data in our data table. Although it was difficult deciphering those elements that possessed contradictory physical and chemical properties, we analyzed our detailed data table and, with our knowledge of the three categories, came to the conclusion that sample elements B and G were metals, sample elements A and E were nonmetals, and sample elements C, D, and F were metalloids.
Procedure:
Even before beginning the procedure, our group, The Acids, spent more time than other groups to organize our notes, data table, element samples, and labels with the motive of conducting a quick, smooth, and accurate experiment. First, we gathered all the tools needed to begin the experiment: two plastic wellplates, a notebook, a pen, a piece of paper, scotch tape for labeling, a hammer-like object, copper (II) chloride, and hydrochloric acid. Then, we waited as Rachel distributed sample elements A-G to each lab group. Before crushing the elements, we waited until all samples were placed on our piece of paper and labeled with their corresponding letters.
We also ripped pieces of scotch tape in half, and labeled two of the same letter on each piece; we attached these pieces of tape to the wellplates, assigning the same letter to each set of two wells. After we were sure we had everything organized, we documented the initial appearances of each sample onto our data table very carefully and in great detail to ensure accurate results. From the data of the appearances alone, sample A appeared to be nonmetallic, samples B and C appeared to be metallic, and samples D, E, F, and G were undecided. Once we were sure everything was organized, we were ready to crush each sample element to decide whether they were malleable or brittle.
Since sample A was originally a powder and remained a powder, samples C , E, and F were brittle, and sample D was very hard to break and sort of brittle, we recorded them as nonmetallic. Because sample B was clearly malleable, we recorded it as a metal. Due to brittleness, yet shiny and hollow chambers revealed in sample G, we recorded it as undecided. After crushing, we divided each sample element in half and placed them side-by-side in the wells in the labeled wellplate.
Rachel tested each crushed sample element’s electrical conductivity with the electrical conductivity apparatus; even if the light bulb was only slightly lit, the light indicated electrical conductivity.
Sample elements A and E were not conductive and sample elements B, C, D, F, and G were conductive—metals conduct electricity. Next, we were ready to fill the wells with copper (II) chloride and hydrochloric acid. We decided to fill the left well of each set with copper (II) chloride, and the right side of each set with hydrochloric acid. After placing 20 drops of each into the wells, we had to wait for a reaction (if any) for three to five minutes.
After three minutes, we analyzed each well set. On the copper chloride side of each sample, the left side of each set, we observed that the liquid simply sat in a pool around each sample for all samples except for sample G. In copper (II) chloride well G, the sample turned black, and a black substance began to come off of the sample. With this data, we recorded only sample G as metallic. On the hydrochloric acid side of each sample, the left right side of each set, again, the liquid sat in a pool around each sample. This time, there were two exceptions in hydrochloric acid well D and, again, well G. The reaction in well D was not very prominent, but we noticed that some bubbles formed in the clear liquid around our sample. In well G, we noticed many bubbles coming off and popping off of our sample that appeared to be dissolving. From this data, we concluded that samples D and G were metallic.
After making sure we had all of our notes down and data table complete, we cleaned up our supplies and went on to analyze the properties of each sample element to reach a conclusion as to what was metallic, nonmetallic, or a metalloid.
Results:
Although this lab may seem simple, our group had some trouble classifying our cumulative data into metal, nonmetal, or metalloid. The metals and nonmetals were very clearly shown in our results from the five observations we produced, but due to contradicting properties, the metalloids were not. Just looking at the initial appearance of our samples to decipher if each sample was a metal or nonmetal was complicated because many samples such as D, E, F, and G contained small particles of shiny bits, or tiny specs of luster. Drawing conclusions from just appearance would often lead to inaccurate data, so we had to use our conclusions from the tests for electrical conductivity, crushing, and chemical reactions with copper (II) chloride and hydrochloric acid as well. Since a lot of the data per sample was contradictory, it made it difficult to decide what category each fit into. In addition, the concept of deciding how a sample could be a metalloid was very unfamiliar to us, so we had to review in our book that although metalloids have properties intermediate to metals and nonmetals, sometimes the absence of a property that defines a metal or nonmetal does not alienate it from being part of one of those categories. After difficult analysis, we concluded that element samples C, D, and F were metalloids, element samples A and E were nonmetals, and element samples B and G were metals.
Data Analysis:
1.Classify each property tested in this investigation as either a physical property or a chemical property.
Appearance: Physical property
Conductivity: Physical property
Crushing: Physical property
Reactivity with copper (II) chloride: Chemical property
Reactivity with acid: Chemical property
2. Sort the seven coded elements into two groups based on similarities in their physical and chemical properties.
1st Group: (Metals) B, G
2nd Group: (Non-Metals) A, C, D, E, F
3. Which element or elements could fit into either group? Why?
Elements C, D, and F could fit into either group—metalloids. These elements could be metalloids because although some of them did or did not show to posses conductivity, some were brittle and did not react with either acid solution, and some did. Because these elements were contradictory in their cumulative results for the tests, they could be classified as metalloids.
4. Using the following information, classify each tested element as a metal, a nonmetal, or a metalloid:
-Metals have a luster, are malleable (can be hammered into sheets), and conduct electricity.
-Many metals react with acids; many metals also react with copper (II) chloride solution.
-Nonmetals are usually dull in appearance, are brittle, and do not conduct electricity.
-Metalloids have some properties of both metals and nonmetals
Metals: Elements B and G.
Nonmetals: Elements A and E.
Metalloids: Elements C, D, and F.
Data Table:
Conclusion:
After having a class discussion, we discovered that we were accurate in almost all of our conclusions based on our data, with two exceptions. We discovered that element A, that we classified as a nonmetal, was aluminum (Al), a metal. This mistake occurred because aluminum is right on the edge of the break between metals and nonmetals, above some metalloids. Because of its closeness to the nonmetals on the periodic table, our mistake in confusing aluminum, a metal, for a nonmetal, was not uncommon. Additionally, the aluminum sample was in powder form, and powder rarely conducts electricity. Our second mistake was with element sample F, Carbon (C). On the periodic table, carbon is a nonmetal, but we classified it as a metalloid. Because carbon conducted electricity but was brittle and did not react with either copper (II) chloride and hydrochloric acid, we thought that since metals conduct electricity, it would be a metalloid. Now we realize that even though carbon conducts electricity, it is a nonmetal. Despite these two errors, we were correct in our conclusions for sample elements B, C, D, E, and G: element B was tin (Sn), a metal, element C was Silicon (Si), a metalloid, element D was Antimony (Sb), a metalloid, and element G was zinc (Zn), a metal. Overall, this lab experiment was very helpful in understanding the differences between metals, nonmetals, and metalloids, and how to characterize elements based on experiments.
Subscribe to:
Posts (Atom)