3SAS #31-38, page 235
31) Name and give the molecular formula for the alkane with a molar mass of
a. 44 g/mol
b. 72 g/mol
a. Propane, C3H8
b. Pentane, C5H12
32) What does -ane imlply about the carbon-carbon bonding in hexane?
The suffix -ane implies that hexane is an alkane.
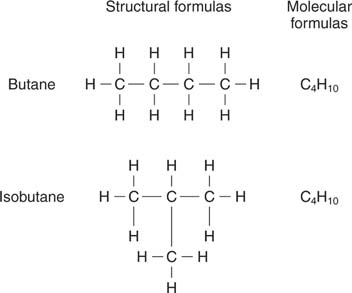
33) Are the following three molecules isomers of one another? Explain your answer. [diagram]
Yes, each of these molecules are isomers of one another. They are all combosed of 5 carbon atoms and 12 hydrogen atoms, but appear to be different because of the arrangement of atoms. These three molecules are structional isomers of one another, for they have identical molecular formulas but different arrangements of atoms.
34) Draw structural formulas for at least three structural isomers of C9H20
See drawings.
35) What is the shortest-chain alkane that can demonstrate isomerism?
Butane (C4H10) is the shortest-chain alkane that can demonstrate isomerism- alkanes with four or more carbon atoms can be demostrated as straight-cchain structures, branched-chain structures, and ring structures.
36) An unbranched hydrocarbon molecule can be represented as a linear chain or as a zig-zag chain. Explain in what way both representations are correct.
Both representations are correct because their different arrangements of atoms does not change the identical molecular formulas of the molecule; this would make the molecules structural isomers of each other.
37)
a. Draw two hexane structural isomers, one a straight-chain molecule and the other a branched-chain molecule.
b. Which of the two isomers you drew would have the lower boiling point? Explain your answer.
a. See drawing.
b. The branched-chain molecule would have the lower boiling point. Since the straight-chain molecule has greater molecule-to-molecule contact, it has a stronger intermolecular force than the branched-chain molecule, resulting in a higher boiling point.
38) Which of each pair of the following hydrocarbon molecules would have the lower boiling point? In each case, describe your reasoning.
a. a short, straight chain or a long, straight chain.
b. a short, branched chain or a long, branched chain.
c. a short, branched chain or a long, straight chain.
a. A short, straight chain would have a lower boiling point because of decreased molecule-to-molecule contact than the longer boiling point. The bonds of this chain would be easier to break than a longer straight chain. However, this straight chain would have a higher boiling point than a branched chain.
b.A short, branched chain would have the lower boiling point. Although the bonds of a branched chain are easier to break than those of a straight chain, it would be more difficult to break more molecular bonds within the long chain, resulting in a higher boiling point.
c. A short, branched chain would have a lower boiling point. Straight chains have stronger intermolecular forces that hold together each molecule in contact, where as bonds between branched chains are more breakable due to the decreased intermolecular molecular forces between them.
3SBS #1-10, page 258
1) From a chemical viewpoint, why is petroleum sometimes considered "buried sunshine"?
Petroleum is sometimes cosidered "buried sunshine" because as a fossil fuel, it originates from biomolecules of prehistoric plants and animals. The energy released by burning petroleum represents energy originally captured from sunlight by these prehistoric green plants during photosynthesis; "buried sunshine".
2) Define and give one example of
a. potential energy
b. kinetic energy.
a. Potential energy is energy of position, or stored energy ready to be released. An example of potential energy is a the energy within an unreleased winded up spring toy.
b. Kinetic energy is energy related to motion. A car rolling down a hill is an example of kinetic energy (after the pedal on a breaked car, representing potential energy, is released).
3) In terms of chemical bonds, what happens during a chemical reaction?
Chemical energy, another form of potential energy, is stored within the bonds in chemical compounds. When an energy-releasing reaction takes place, the bonds break and reactant atoms reorganize to form new bonds and release energy. If more energy is released than originally started with, the reaction is exothermic, but if less energy is released than originally started with, the reaction is endothermic.
4) Based on its structural formula, which has more potential energy, a molecule of methane or a molecule of butane? Explain your answer.
A molecule of butane has more potential energy, it has more carbons than methane and a higher boiling point than methane. The bonds of butane are harder to break than the bonds of methane, resulting its higher potential energy.
5) Classify each of the following as primarily a demonstration of kinetic energy or potential energy:
a. a skateboard positioned at the top of a hill.
b. a charged battery in a flashlight that's turned off.
c. a rolling soccer ball.
d. gasoline in a parked car.
e. water flowing over a waterfall.
a. Potential energy.
b. Potential energy.
c. Kinetic energy.
d. Potential energy.
e. Kinetic energy.
6) Why is energy required to break chemical bonds?
Energy is required to break chemical bonds because it is what causes the reactant bonds to break and reorganize to form new bonds and energy.
7) For each of the following events, determine whether the reaction is exothermic or endothermic. Explain your answers in terms of bond breaking and bond making:
a. burning wood in a campfire.
b. cracking large hydrocarbon molecules.
c. digesting a candy bar.
a. Exothermic. More energy is released than is required to begin the chemical reaction.
b. Endothermic. More energy is required to crack large hydrocarbon molecules than is released.
c. Endothermic. It takes more energy to digest a candy bar than the energy released after digestion.
8) Burning a candle is an exothermic reaction. Explain this fact in terms of the quantity of energy stored in the bonds of the reactants compared with the quantity of energy stored in the bonds of the products.
The product of a burning candle yields more energy than the energy to begin the reaction with an unlit candle. Since more energy is let off than required to begin the reaction, burning a candle is an exothermic reaction.
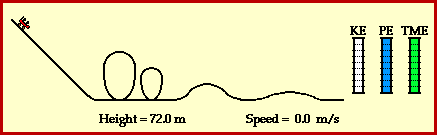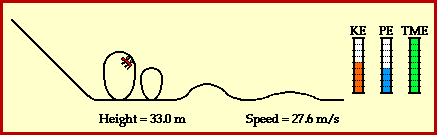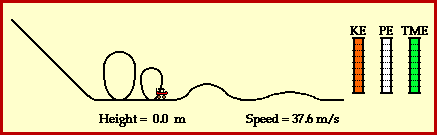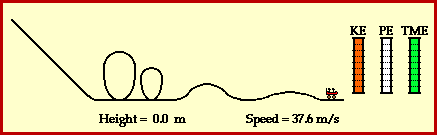
9) Using Figure 3.24 on page 240 as a model, draw a potential energy diagram that illustrates the energy change when hydrogen gas reacts with oxygen gas to produce water and thermal energy.
See drawing.
10) State the law of conservation of energy.
The law of conservation of energy states that energy is neither created nor destroyed in any mechanical, physical, or chemical processes.
A large number of polypropylene dispersions film manufacturers are involved in manufacturing this kind of film which offers enhanced clarity and found perfect for production with pictures and words such as toys.
ReplyDeleteGood information, also join suppliers community at
ReplyDeletehttps://directsupplier.org/chemicals
and boost sales
Spacecrafts: someday Oxygen will be obtained from CO2 too
ReplyDeleteThis comment has been removed by the author.
ReplyDelete(1)...space-elevator (orbital station ramp: fresh air)... CO2 + laser UV → C + O2... RENOVATOR AIR UNIT: Powerful-Compressør³ continuously extracting the air Only from bathrooms ((((air passes condenser metallic grid which has a cold extreme ship´s wall through at outer space, water drops distilled go out centrifuged...air´s pipe goes to outer space sufficient for air cooling only till CO2 freezing point, their already solid ice particles go away into the air current centrifuged impacting against the wall of *U-Tube* of the air which *has exit in the U zone* to the roomy hollow on lateral wall of a *Cylinder-Tube*, perpendicular at 90º, that has a, with solid axis at both sides running through all components, ↔▬╠▬▬|PISTON|▬▬╣▬↔ *shoving the ice pieces at both ways goes↔comes*; for isolate air current the same PISTON *at both sides* opens↔closes Cylinder extremes on both *intersections* with U-Tube, and shoves/releases *over extreme of this central now hollow axis* (length="T"╣branch-line´s ø) of a ═|Circular Cap valve, how a mushroom with its stem hollow, which has on the back side a *spring* against cavity (for spring+Cap) in the wall of *another perpendicular Tube forming "T" at both sides*, 2 branch-lines, of the ╠*Cylinder-Tube*╣, from that another Tube *arrives from one side↑* hot CO2 current, this *drags towards the another side* and thaws the ice...from both ╠branch-lines╣ which outlet into 1 only tube, gas CO2 passes now by a thin and long tube with mirror interior walls ╩╩╩╩╩╩ with inclined multiples UV laser rays discharges...finally C and separation of O2 which passes to ■Tank reservoir O2 liquid))))... (Similar appropriate procedures for other processes of cooling and liquefaction, hot water and air with solar energy) → compressed dirty air through anti-return retention´s ÷valve goes to 2 ©Deposits at outer space...besides punctually reinforcing, when sensor detects presence into bathroom connects extractor ~Ventilator of Pressure (220 v.ac/50 W) that carries the dirty air, already without water nor CO2, to a graphene´s inflatable ºBalloon at outer space, another Compressør² extracts the air of the ºBalloon and injects it compressed through another anti-return retention´s ÷valve to circuit of the ©Deposits which have the dirty air already liquid at cryogenic temperature → #DISTILLER Linde mini → N and O2 ((someday will be obtained also Oxygen from (California University)...CO2 + laser UV ultra-short wave → C + O2...electrons of CO2 molecule are in covalent bond, shared, they acquire energy from photons of the UV laser jumping thus to higher orbits and breaking thus the molecule)), the dirty air remains that can´t be used is thrown to space...the 2 ©Deposits alternatively working, one closed exit mode distilling, another open exit mode throwing; both have at entrance a solenoid┴valve, and exit´s hinged door slightly open/close with 1 motorized gyratory screw outside the ©Deposit → █Tank Reservoir Air Liquid that receives, if it is necessary, also O2 from ■Tank reservoir O2 liquid... From █Tank Reservoir Air Liquid (N+O2) arrives more/less new air for maintaining the ambient pressure: »»» breeze of clean fresh air 1 bar »»»░... Finally shall smell to roses in a Spacecraft.
ReplyDelete(1b)...space-elevator (orbital station ramp: fresh air)... increasing wind-speed/centrifugal-force ice particles into *spiral@pipe now with endless closed circuit*: 1 *exit towards compressor*, and 1 *entrance* air ship with ventilator of pressure sending air to circuit and *deflector* anti↓exit cold dirty air that continues forward by inertia → (occasionally, if it is necessary, the system is stopped for eliminate ice adherences insufflating hot air)... Circuit inside *1 axial-turbine*, the same as jet-engine, accelerating air that endless gyrates into circuit, with its *motor outside the tube* gyration sending with transmission | axis perpendicular to the turbine--axis.
ReplyDelete...3d bioprinting-Immortality (biological timers: reverse aging)… old age short telomere, Youth Long Telomere...ON... Immortality, here we go...goooooo!>>… modifying Biological Timers for every birthday to reach 1 less year old on biological age...until arrive to the 18, then come back to reach 1 more until arrive to the 50, then backward again...and so for all Eternity… until arrives an accident: your recorded Memory to the new young perfect identical Bioprinted Body and...Immortality...already the accidents do not matter.
ReplyDeleteThis comment has been removed by the author.
ReplyDeletevery interesting and useful blog. If you are interested in chemistry, check out this blog about silicon chemistry
ReplyDeletehttps://silsesquioxane.blogspot.com/
ReplyDeleteVery Nice Post
To read physics
To read chemistry
To read biology
Nice Post,
ReplyDeleteOur Chemistry homework helpers have access to a scholarly database which they use to find out the most useful & relevant information for your paper to provide you the best assignment help online.
...interstellar travel constant acceleration (without "God bless to the United States of America")... and to the rest of the World that crack to it a ray... Only USA cans do this: leave to be USA and take the lead of UNION WORLD without nations, without wars and without frontiers (cities only) with 1 only World Government, 1 only Idiom, 1 only Army, 1 only Anthem and 1 only Earth Flag, admitting in Equality in UW to all countries that also want leave to be it and go away together toward the Immortal Future without gods religion "blesses" nor tales, instead of continue wasting the time raising barriers and with the demented religious-patriots assail-Congresses who make a country in which the house of the president is called the "white" house... Come on to the Future, and goodbye UN... Hello UW...
ReplyDeleteLeading economies (Germany, Japan, South Korea, Italy and the Czech Republic) in which high tech industries contribute an immense volume in economic development are vulnerable to the industrial policy of China. software engineering assignment help
ReplyDeleteessay writing help
buy book report online
ireland assignment help
malyasia assignment help
You can always trust the experts of MyAssignmentHelpAu for your needs of assignment writing services. Being the best and most reliable assignment help Los Angeles service providers in the business, they ensure that you get all what you need to achieve the best grades! Have your task completed to perfection with them!
ReplyDeleteThis comment has been removed by the author.
ReplyDeletelinkedin
ReplyDeletelinkedin
linkedin
tlinkedin
linkedin
linkedin
Hi there,
ReplyDeleteThank you so much for the post you do and also I like your post, are you looking for Buy Chemical Online in the whole USA? We are providing Buy chemical products Online, Buy Research Chemical Online, Buy Ecstasy Online, Buy good cocaine, Buy MDMA Online, Buy LSD Online, Buy Pure Caffeine Online, LSD, MDMA, ,Cocaine, Buy Research Chemical Online, Buy Cocaine Online, Contact Us Buy Chemical Online, BUY 100% PURE CAFFEINE POWDER ONLINE, Buy LSD crystal Powder Online, Buy MDMA Crystal Online, BUY MDMA POWDER CRYSTALS ONLINE, Buy Cocaine Online, BUY 3-CMC CRYSTAL ONLINE,BUY 5F-ADB ONLINE, BUY A-PVP CRYSTAL ONLINE,BUY ACETYL FENTANYL ONLINE, BUY AMPHETAMINE ONLINE, BUY BMK OIL ONLINE,BUY BZP (BENZYLPIPERAZINE) POWDER ONLINE,BUY FENTANYL POWDER ONLINE, Buy Ketamine Powder Online, BUY 25I-NBOME ONLINE,BUY 3-CMC CRYSTAL ONLINE,BUY 3-MMC ONLINE,BUY AMPHETAMINE ONLINE in the world with the well price and our services are very fast.
Click here href=" https://www.biochemspharma.com/"> title=”Buy Chemical Online | Buy Pure Caffeine Online | LSD, MDMA, Cocaine”
MORE DETAILS......
Contact us at: +1(858)-480-1436
Email: info@biochemspharma.com
Very Nice Post. This post is very helpful. Thanks.
ReplyDeleteRefrigerated Transport – Texas provides crucial temperature-controlled logistics for perishable goods, guaranteeing the preservation of quality and freshness from origin to destination. It supports various industries, including agriculture, food production, and pharmaceuticals, ensuring the safe delivery of sensitive products.
ReplyDeleteCmolds is a top app design company renowned for its exceptional expertise in crafting innovative and user-centric mobile applications. With a track record of excellence, Cmolds consistently delivers cutting-edge solutions that redefine the mobile app landscape. Trust Cmolds as your premier partner for top-tier app design and development services.
ReplyDeleteRamma Foundation Repair is your trusted source for Foundation Repair Edmonton. We provide expert solutions to safeguard your home's foundation.
ReplyDeleteStop stressing about your assignment anymore. A Global assignment expert is here to help you with all your assignment. Global assignment help writes assignment plagiarism free. Assignment expert motive is to write assignment with accuracy and deliver it on time. Thesis assignment help offers assignment services in low budget. Our team of experts will write assignment and also help you to understand assignment. Do visit our managerial website for more queries.
ReplyDeleteChemistry is such a fascinating subject, especially when exploring the intricacies of chemical reactions and the periodic table! For students looking to deepen their understanding or prepare for their exams, it’s essential to find the right resources. If you need to streamline your study process, consider platforms that allow you to take my exam online . This can provide flexibility and help you focus on mastering the concepts
ReplyDeleteChemistry can be a challenging subject for many students, especially when trying to grasp complex concepts like organic reactions, atomic structures, or thermodynamics. For those balancing multiple responsibilities, it can be overwhelming. If you're finding it hard to keep up with your chemistry assignments or exams, you might want to consider hiring an online class taker . This option allows you to get expert help, ensuring you stay on top of your studies while managing your other commitments efficiently
ReplyDeleteThis material made me comprehend isomerism and molecular structures better. It was clearly written and organized. Being busy, I really appreciate the simplicity. I'd certainly refer Hire Exam Nerds to anyone considering, Should I pay to take my online exam? they make academic achievement possible and hassle-free.
ReplyDeleteChemistry is such an interesting subject, particularly when delving into the nitty-gritty of chemical reactions and the periodic table! For students who wish to further learn or review for their exams, it's critical to get the proper resources. If you need to simplify your study process, consider sites where you can take my exam for me. This can give you the flexibility and enable you to focus on learning the concepts
ReplyDeleteThis comment has been removed by the author.
ReplyDeleteChemistry Blog opens a world where science feels alive and exciting. Trusted Advisor providing best articles like entertainment, celebrity, scandals, drama, lifestyle, technology, health makes it even more engaging for readers. Truly a perfect space for learners and enthusiasts alike!
ReplyDeleteYour work in this section is truly impressive and demonstrates a strong understanding of the topic. Each answer is written clearly and with thoughtful detail. In the middle of this effort, it’s worth mentioning that Eco Liv is providing the best deals and discount codes on electronics, pets, jewellery, travel, gifts, clothing & apparel, and more, which reflects how awareness of such platforms can enhance practical knowledge in real-life situations.
ReplyDelete...interstellar travel constant acceleration (global solar energy without accumulation)... cover Deserts with Solar Panels aloft (beneath them the ground improves): from the S☼L→ to the Shad☺w of the rotating Planet, reciprocal Global sending of Electricity via Submarine Cables. Without "those" dam patriots against the Progress of Humanity cutting the Cables.
ReplyDelete...▓ ▓ (tonyon - Antonio Iglesias Noja - project GSENA: Global Solar Energy No Accumulation) ▓ ▓...
DeleteFrom 3sas-31-38-page-235-3sbs-1-10, we see key insights that connect knowledge with everyday life. In the middle, Trusted brands providing fashion, clothes, shopping, shoes, makeup, skin, beauty, and more show how quality and style shape our choices. Overall, it highlights the importance of making informed and practical decisions.
ReplyDelete...interstellar travel constant acceleration (tonyon hypothesis)... "The orbital speed of electrons varies inversely to the acceleration", whether gravitational or motional. That´s why on Mars, with less gravitational acceleration, an atomic clock runs faster than on Earth. Is the clock running fast or slow, not "Time".
ReplyDelete(3)...interstellar travel constant acceleration (tonyon hypothesis)... It is based on: the electrons have mass and they are affected by the acceleration, changing not "the Time" but its measurement. Orbital change: Electrons descend emitting energy, angular velocity increases, linear velocity decreases. Electrons ascend absorbing energy, angular velocity decreases, linear velocity increases. ("The orbital speed of electrons varies inversely to the acceleration", gravitational or motional). In a mechanic or electric clock, increased acceleration decreases the speed of its mechanism, even stopping it if acceleration is extreme... 6 synchronized atomic clocks, 2 remain on Earth: 2 spacecrafts ready for launch, 2 clocks on each spacecraft. One ship enters in Earth orbit to-rotating, the other counter-rotating. Upon ascending, gravitational acceleration decreases in both, but the motional acceleration required to reach orbital speed is much greater than gravitational acceleration, causing the 4 clocks DURING the acceleration to run slower than the 2 on Earth. The to-rotating ship has accelerated less to orbit, so its clocks ran faster than the 2 on the other ship. In the counter-rotating ship, vice versa in everything. (If already in orbit "without further acceleration", its clocks were synchronized again with the clocks on Earth, in orbit, in the Space, with less gravitational acceleration, its clocks would run faster than the clocks on Earth. So: it´s not that "the greater the speed, the more Time slows down", it´s that to reach that greater speed, it´s necessary to accelerate more for a period of time, and that´s when the clock slows down, not "the Time")... On the surface of Mars, with less gravitational acceleration: the 2 clocks run faster that the 2 on Earth, but on the round trip, during the brief period of greatest acceleration in the ship, its 2 clocks ran slower than the 2 on Earth... Black hole: the ship approaches... gravitational acceleration increases, the ship speeds ever faster towards "the hell", and its 2 clocks run slower and slower than before. The ship turns 180º and moves away towards "the salvation", now gravitational acceleration is decreasing, and the ship´s motional acceleration must be increased to escape (if it still can...), during each change, its 2 clocks, due to the differential of both accelerations, run faster or slower than before... When the ship finally returns to Earth, its crew members are neither younger nor older than "the earthlings"; it is the clocks that have operated at different speeds according to the accelerations. The 6 atomic clocks remain synchronized only in each different pair, if they have functioned correctly. (AI: "how an atomic clock works").
Delete(3a)...interstellar travel constant acceleration (tonyon hypothesis)... with the future Gravitational Transformers: what would happen if... Starship at 1 Million G... Habitable areas: 1M-g constant acceleration towards the ground ↓↓engines↓↓ (-) 999 999g constant acceleration towards the ceiling ↑↑gravitational transformers↑ = 1g constant acceleration towards the ground↓... Habitable areas: 2 atomic clocks outside and 2 inside. The 2 inside operate at 1g normally. The 2 outside at 1M-g... perhaps their electrons would be stopped in their orbits: the 2 clocks outside would stop, not "the Time" outside would stop and inside wouldn´t. If the electromagnetic attraction proton/electron were stronger than the forces of the enormous acceleration on the electrons, the electrons would fall on the atomic nucleus, thus the atoms are left with their electrons stopped and glued to the nucleus... Without the valence electrons already there are no molecules... What would happen to the structure of the Ship ("that is the Matter, mostly empty space") already the Matter formed by atoms with the electrons stopped in their orbits, or by atoms with their electrons stopped and glued to the nucleus?... the ship´s structure would collapse like sand without cement... To avoid the "acceleration effect" place the Gravitational Transformers throughout the ENTIRE structure of the Ship, not just in the habitable areas. This way, the entire Ship experiences 1g even traveling at 1M-g, (1Mega-g).
DeleteThe best forex trading platforms in India provide traders with advanced charting tools, real-time market data, fast execution, and a user-friendly interface. Platforms like MetaTrader 4 (MT4) and MetaTrader 5 (MT5) are widely preferred for their reliability, technical indicators, and automated trading features. A good trading platform helps Indian traders analyze the market effectively, manage risk efficiently, and trade with confidence in the global forex market.
ReplyDeleteContact us Address – 1st Floor, The Sotheby Building, Rodney Bay, Gros-Islet, SAINT Lucia P.O Box 838, Castries, Saint Lucia Phone no – +97144471894 Website – https://winprofx.com/
Forex trading companies in India include a mix of SEBI-registered brokers that allow legal currency derivatives trading on recognized exchanges and financial firms involved in foreign exchange services. Well-known Indian brokers like Zerodha, Upstox, Angel One, ICICI Direct, HDFC Securities, Kotak Securities, Sharekhan, Motilal Oswal, and 5Paisa are authorized by the Securities Exchange Board of India (SEBI) to offer INR-based forex futures and options on exchanges like the NSE and BSE, giving traders access to USD/INR, EUR/INR, GBP/INR and JPY/INR pairs within the regulatory framework. Additionally, companies such as WSFx Global Pay provide foreign exchange services and prepaid forex products under Reserve Bank of India (RBI) authorization, serving travel, remittance, and cross-border payment needs.
ReplyDeleteContact us Address – 1st Floor, The Sotheby Building, Rodney Bay, Gros-Islet, SAINT Lucia P.O Box 838, Castries, Saint Lucia Phone no – +97144471894 Website – https://winprofx.com/