3SAS #31-38, page 235
31) Name and give the molecular formula for the alkane with a molar mass of
a. 44 g/mol
b. 72 g/mol
a. Propane, C3H8
b. Pentane, C5H12
32) What does -ane imlply about the carbon-carbon bonding in hexane?
The suffix -ane implies that hexane is an alkane.
33) Are the following three molecules isomers of one another? Explain your answer. [diagram]
Yes, each of these molecules are isomers of one another. They are all combosed of 5 carbon atoms and 12 hydrogen atoms, but appear to be different because of the arrangement of atoms. These three molecules are structional isomers of one another, for they have identical molecular formulas but different arrangements of atoms.
34) Draw structural formulas for at least three structural isomers of C9H20
See drawings.
35) What is the shortest-chain alkane that can demonstrate isomerism?
Butane (C4H10) is the shortest-chain alkane that can demonstrate isomerism- alkanes with four or more carbon atoms can be demostrated as straight-cchain structures, branched-chain structures, and ring structures.
36) An unbranched hydrocarbon molecule can be represented as a linear chain or as a zig-zag chain. Explain in what way both representations are correct.
Both representations are correct because their different arrangements of atoms does not change the identical molecular formulas of the molecule; this would make the molecules structural isomers of each other.
37)
a. Draw two hexane structural isomers, one a straight-chain molecule and the other a branched-chain molecule.
b. Which of the two isomers you drew would have the lower boiling point? Explain your answer.
a. See drawing.
b. The branched-chain molecule would have the lower boiling point. Since the straight-chain molecule has greater molecule-to-molecule contact, it has a stronger intermolecular force than the branched-chain molecule, resulting in a higher boiling point.
38) Which of each pair of the following hydrocarbon molecules would have the lower boiling point? In each case, describe your reasoning.
a. a short, straight chain or a long, straight chain.
b. a short, branched chain or a long, branched chain.
c. a short, branched chain or a long, straight chain.
a. A short, straight chain would have a lower boiling point because of decreased molecule-to-molecule contact than the longer boiling point. The bonds of this chain would be easier to break than a longer straight chain. However, this straight chain would have a higher boiling point than a branched chain.
b.A short, branched chain would have the lower boiling point. Although the bonds of a branched chain are easier to break than those of a straight chain, it would be more difficult to break more molecular bonds within the long chain, resulting in a higher boiling point.
c. A short, branched chain would have a lower boiling point. Straight chains have stronger intermolecular forces that hold together each molecule in contact, where as bonds between branched chains are more breakable due to the decreased intermolecular molecular forces between them.
3SBS #1-10, page 258
1) From a chemical viewpoint, why is petroleum sometimes considered "buried sunshine"?
Petroleum is sometimes cosidered "buried sunshine" because as a fossil fuel, it originates from biomolecules of prehistoric plants and animals. The energy released by burning petroleum represents energy originally captured from sunlight by these prehistoric green plants during photosynthesis; "buried sunshine".
2) Define and give one example of
a. potential energy
b. kinetic energy.
a. Potential energy is energy of position, or stored energy ready to be released. An example of potential energy is a the energy within an unreleased winded up spring toy.
b. Kinetic energy is energy related to motion. A car rolling down a hill is an example of kinetic energy (after the pedal on a breaked car, representing potential energy, is released).
3) In terms of chemical bonds, what happens during a chemical reaction?
Chemical energy, another form of potential energy, is stored within the bonds in chemical compounds. When an energy-releasing reaction takes place, the bonds break and reactant atoms reorganize to form new bonds and release energy. If more energy is released than originally started with, the reaction is exothermic, but if less energy is released than originally started with, the reaction is endothermic.
4) Based on its structural formula, which has more potential energy, a molecule of methane or a molecule of butane? Explain your answer.
A molecule of butane has more potential energy, it has more carbons than methane and a higher boiling point than methane. The bonds of butane are harder to break than the bonds of methane, resulting its higher potential energy.
5) Classify each of the following as primarily a demonstration of kinetic energy or potential energy:
a. a skateboard positioned at the top of a hill.
b. a charged battery in a flashlight that's turned off.
c. a rolling soccer ball.
d. gasoline in a parked car.
e. water flowing over a waterfall.
a. Potential energy.
b. Potential energy.
c. Kinetic energy.
d. Potential energy.
e. Kinetic energy.
6) Why is energy required to break chemical bonds?
Energy is required to break chemical bonds because it is what causes the reactant bonds to break and reorganize to form new bonds and energy.
7) For each of the following events, determine whether the reaction is exothermic or endothermic. Explain your answers in terms of bond breaking and bond making:
a. burning wood in a campfire.
b. cracking large hydrocarbon molecules.
c. digesting a candy bar.
a. Exothermic. More energy is released than is required to begin the chemical reaction.
b. Endothermic. More energy is required to crack large hydrocarbon molecules than is released.
c. Endothermic. It takes more energy to digest a candy bar than the energy released after digestion.
8) Burning a candle is an exothermic reaction. Explain this fact in terms of the quantity of energy stored in the bonds of the reactants compared with the quantity of energy stored in the bonds of the products.
The product of a burning candle yields more energy than the energy to begin the reaction with an unlit candle. Since more energy is let off than required to begin the reaction, burning a candle is an exothermic reaction.
9) Using Figure 3.24 on page 240 as a model, draw a potential energy diagram that illustrates the energy change when hydrogen gas reacts with oxygen gas to produce water and thermal energy.
See drawing.
10) State the law of conservation of energy.
The law of conservation of energy states that energy is neither created nor destroyed in any mechanical, physical, or chemical processes.
Chemistry Blog
Wednesday, July 20, 2011
Extra Credit for Friday, July 22: Stress and ageing: A question of attitude: The link between chronic stress and a marker of old age is being disentangled
Stress and ageing
A question of attitude
The link between chronic stress and a marker of old age is being disentangled
Apr 7th 2011 | from the print edition
Chromosomes, which are located in white blood cells, replicate themselves as the cells they are in divide. The telomeres, which stop the chromosomes from "fraying", shorten every time the chromosome is divided. According to the Hayflick limit, telomeres can only divide 50-70 times; after this many divisions, the chromosome is unable to divide any further. Although it is a good thing that when quickly dividing cells in a tumor hit the Hayflick limit they stop dividing completely, reaching the Hayflick limit is also an indication of old age. It is not good for this halt in dividing to happen prematurely, for tissues, like the tissues in the immune system, have to be dividing constantly in order to function properly. Chronic stress, defined as the response to emotional pressure suffered for a prolonged period over which an individual perceives he or she has no control over, such as from suffering from an illness, is known to cause premature shortening of the telomeres. A group of researchers led by Edward Nelson of the University of California, Irvine, at a meeting of the American Association for Cancer Research, answered a question that has been unanswered: Is premature shortening of the telomeres irreversible? Although the results must be treated carefully, the research Edward Nelson and his team conducted seems to show that stress management is the key; it stops telomeres from shortening AND it promotes their repair. In order to reach this conclusion, a study Nelson and his group of researchers involved counseling women who suffered from chronic stress due to cervical cancer with telephone counseling, and of course, a control group of women who did not receive counseling. Although the women who were counseled still had cervical cancer, they reported that the quality of their lives had improved, as well as the strengths of their immune systems. In order to analyze these results, Dr. Nelson examined the white blood cells of the women who endured counseling. He found that counseling reset their Hayflick countdowns; the counseling not only stopped the shrinkage of their telomeres, but promoted their growth! Elizabeth Blackburn of the University of California, San Francisco, supported Dr. Nelson's results by showing that like counseling, exercise also has a similar resetting effect on the telomeres with her Nobel Prize winning discovery of the enzyme that repairs telomeres. Dr. Nelson's work should be replicated in order to contribute to an expansion of knowledge of the relationship between the mind and the body, but from his results, it is safe to say that positive attitude not only helps the state of mind, but the health of the body as well.
http://www.economist.com/node/18526881
A question of attitude
The link between chronic stress and a marker of old age is being disentangled
Apr 7th 2011 | from the print edition
Chromosomes, which are located in white blood cells, replicate themselves as the cells they are in divide. The telomeres, which stop the chromosomes from "fraying", shorten every time the chromosome is divided. According to the Hayflick limit, telomeres can only divide 50-70 times; after this many divisions, the chromosome is unable to divide any further. Although it is a good thing that when quickly dividing cells in a tumor hit the Hayflick limit they stop dividing completely, reaching the Hayflick limit is also an indication of old age. It is not good for this halt in dividing to happen prematurely, for tissues, like the tissues in the immune system, have to be dividing constantly in order to function properly. Chronic stress, defined as the response to emotional pressure suffered for a prolonged period over which an individual perceives he or she has no control over, such as from suffering from an illness, is known to cause premature shortening of the telomeres. A group of researchers led by Edward Nelson of the University of California, Irvine, at a meeting of the American Association for Cancer Research, answered a question that has been unanswered: Is premature shortening of the telomeres irreversible? Although the results must be treated carefully, the research Edward Nelson and his team conducted seems to show that stress management is the key; it stops telomeres from shortening AND it promotes their repair. In order to reach this conclusion, a study Nelson and his group of researchers involved counseling women who suffered from chronic stress due to cervical cancer with telephone counseling, and of course, a control group of women who did not receive counseling. Although the women who were counseled still had cervical cancer, they reported that the quality of their lives had improved, as well as the strengths of their immune systems. In order to analyze these results, Dr. Nelson examined the white blood cells of the women who endured counseling. He found that counseling reset their Hayflick countdowns; the counseling not only stopped the shrinkage of their telomeres, but promoted their growth! Elizabeth Blackburn of the University of California, San Francisco, supported Dr. Nelson's results by showing that like counseling, exercise also has a similar resetting effect on the telomeres with her Nobel Prize winning discovery of the enzyme that repairs telomeres. Dr. Nelson's work should be replicated in order to contribute to an expansion of knowledge of the relationship between the mind and the body, but from his results, it is safe to say that positive attitude not only helps the state of mind, but the health of the body as well.
http://www.economist.com/node/18526881
Final Blog Posting topic: What I got out of summer Chemistry.
I feel as though the hard work I put into the class and hours of homework and studying I have done throughout the six weeks of Chemistry has paid off with my new knowledge and understanding of serious issues the earth faces, the earth's systems, matter, the periodic table, and how things really work. Although sometimes it has been difficult to learn so much condensed information in a short period of time, I feel as though by taking this course, not only have I gained an especially good understanding of concepts involved, but have applied and improved my concentration skills with my diligent and complete work. In first semester, my major take away from the course was the basis is unit conversions, concepts involving the periodic table including properties of the elements and ions, and the importance of conservation of water due to horrible shortages and pollution throughout the world. Not only do I feel as though I grasped the magnitude of this problem, but went beyond the lab experiments we conducted and studied methods of purification of water and ways to conserve. During second semester, I learned more mathematical concepts, more about the construction of atoms and metals, as well as subcategories such as covalent bonds, allotropes, isotopes, and alloys, and more worldly issues such as pollution and global warming. Building off of conversions covered in first semester, during week 5 we were introduced to complicated conversions involving moles and molar masses. We did helpful labs to help us learn about physical and chemical changes of metals as well as ways to change them into alloys. Finally, we learned about the carbon cycle and how solar radiation is transmitted to earth; surprisingly to me, this cycle involved the hydrologic cycle that we covered in first semester. I have discovered that although we learned a vast amount of topics during the six weeks of the Chemistry course, plenty of the information we learned intertwined in a sense as we advanced in the class. Overall, Summer Chemistry has been a rewarding experience for me, and I am glad I spent six weeks of my summer that has, and will continue to benefit me as a high school student.
Tuesday, July 19, 2011
3SAS #1-30 (except for #2, #4, #9, #14, #24, and #25), pages 233-235
1) What is a hydrocarbon?
A hydrocarbon is a molecular compound that only contains atoms of hydrogen and carbon.
3) What characteristics of petroleum make it a valuable resource?
Since petroleum is not only a nonrenewable resource, but a very versatile material that is used both as fuel that can be converted to gasoline, can be used as heat sources, can deliver energy to generate electricity, can generate energy, and is also used to produce useful every day products, it is a valuable resource.
5) What is meant by saying that oil is crude?
Oil is crude because it is pumped from underground and cannot be used in its natural state without some degree of refinement where it is separated into simpler mixtures (through fractional distillation).
6) On average, the United States uses about 20 million barrels of petroleum daily:
a. What is the average number of barrels of petroleum used daily in the United States for building (nonfuel) purposes?
b. How many barrels of petroleum, on average, are burned as fuel daily in the United states?
a. 0.11 x 20,000,000= 2,200,000 barrels
b. 0.89 x 20,000,000= 17,800,000 barrels
7) Name several fuels obtained from crude petroleum.
Heating and cooking fuel, petrochemicals, kerosene, refined oils, gas oil, heavy furnace oil, diesel fuel oil, lubricating oil and grease, heavy oils and wax, cracking stock, petroleum jelly, road oils and asphalt, petroleum coke.
8)
a. List four household items made from petroleum.
b. What materials could be substituted for each of these four household items of petroleum were not available to make them?
a. Water bottle, sports equipment, clothing, artificial limbs.
b. A water bottle can be made out of aluminum, bamboo can be used to make light, flexible, and durable sports equipment, clothing can be made of cotton, and artificial limbs can be made of iron (although it is much better to make them out of plastic).
10)
a. Which world region has the most petroleum reserves relative to its population?
b. Which region has the least petroleum reserves relative to its population?
a. The Middle East has the most petroleum reserves relative to its population.
b. Central Asia, Far East, and Oceania have the least petroleum reserves relative to its population.
11)
a. Which regions consume a greater proportion of the world's supply of petroleum than they possess?
b. Which regions consume a smaller proportion of the world's supply of petroleum than they possess.
a. North America, Central Asia, Far East, and Oceania, Western Europe, and Eastern Europe consume a greater proportion of the world’s supply of petroleum than they possess.
b. The Middle East, Africa, and Central and South America consume a smaller proportion of the world’s supply of petroleum than they possess.
12) Under what conditions could density be used to separate two different liquids?
Density can be used to separate two different liquids if the substances are insoluble with each other.
13) Referring to Table 3.1 (page 216), a mixture of which two of the substances listed would be the easiest to separate from each other by distillation? Explain your reasoning.
Water and acetone would be the easiest to separate from each other by distillation. This is because since water has the highest boiling point, and acetone has the lowest of the four substances, water and acetone would separate fairly quickly and easily by distillation.
15) Referring to Table 3.1 (page 216), sketch a graph of the distillation of a mixture of acetone and water. Label its key features.
16) How does fractional distillation differ from simple distillation?
Fractional distillation does not separate each compound in crude oil, but produces several distinctive mixtures, called fractions. Fractional distillation is also a continuous process. The crude oil is heated in a furnace and then pumped into the fractionating tower. The temperature within the tower (distilling column) is highest at the bottom and decreases towards the top. Smaller and lighter molecules that have lower boiling points vaporize to the top of the tower, and fractions with larger molecules with higher boiling points condense back to liquid in trays in the lower part of the column. Substances with the highest boiling points never vaporize, but drain from the base—bottoms.
17) Petroleum fractions include light, intermediate, and heavy distillates and residues. List three useful products derived from each of these three fractions.
Light: Aviation gasoline and motor gasoline, kerosene, and refined oils.
Intermediate: Gas oil, petrochemicals, heavy furnace oil, and diesel fuel oil.
Heavy: Lubricating oil and grease, heavy oils and wax, and cracking stock.
18) Where in a distillation tower--top, middle, or bottom--would you expect the fraction with the highest boiling point range to be removed? Why?
The fraction with the highest boiling point range would be removed at the bottom. This is because they are thick (viscous) liquids that never vaporize, called bottoms; they drain from the tower’s base.
19) After a fractional distillation, each fraction is still a mixture. Suggest a way to further separate the components of each fraction.
A way to further separate the components of each fraction would be through distillation after a fractional distillation.
20) Rank the following straight-chain hydrocarbons from their lowest boiling point to their highest: hexane (C6H14), methane (CH4), pentane (C5H12), and octane (C8H18). Explain your ranking in terms of intermolecular forces.
Methane (CH4), pentane (C5H12), hexane (C6H14), octane (C8H18).
The boiling points increase with greater amounts carbon atoms within the straight-chains of hydrocarbons; the higher the boiling point, the stronger the intermolecular forces are between the molecules.
21) What is a covalent bond?
A covalent bond is the sharing of two or more valence electrons between two atoms, allowing both atoms to fill their outer shells completely.
22) Why do atoms with filled outer electron shells not form covalent bonds?
Atoms with filled electrons (8 valence electrons) are particularly stable, and therefore, tend to be chemically uncreative. Noble gases are atoms with filled outer electron shells.
23) It has been suggested that a covalent bond linking two atoms is like two dogs tugging on the same sock. Explain how this analogy describes the way that shared electrons hold together atoms in a covalent bond.
Since the two dogs desire the sock, they must share it, although they desire to have it for themselves; like repelling electrons, the dogs pull away from each other, but are still connected by the bond they share with the sock connecting them.
26)
a. What information does a structural formula convey that a molecular formula does not?
b. In what ways is a structural formula an inadequate representation of an actual molecule?
a. A structural formula shows the makeup of a molecule, as well as how high the boiling point is, where as a molecular formula just shows the amount of atoms each element in the formula possesses.
b. The structure of a formula shows how strong molecular bonds within the formula is, as well as the boiling point of the formula.
27) Choose a branched six-carbon hydrocarbon molecule.
a. Draw a Lewis dot structure to represent its structure.
b. Draw a structural formula for the same molecule.
a & b: see drawings.
28) Each carbon atom has six total electrons. Why, then, does the electron-dot representation of a carbon atom show only four dots?
The electron-dot representation of a carbon atom only shows four dots because the four dots represent the valence electrons, located on the outer (and not inner) shell of the atom, where two electrons are located.
29) Use the general molecular formula to write the molecular formula for an alkaline containing
a. 9 carbons.
b. 16 carbons.
c. 10 carbons.
d. 18 carbons.
a. C9H20
b. C16H34
c. C10H22
d. C18H38
30) Calculate the molar mass of each alkane listed in Question 29.
a. 128g
b. 226g
c. 142g
d. 254g
Monday, July 18, 2011
Unit 3, B.3 #1-4, page 344 & 4SBS #14-20, page 361
Unit 3, B.3 #1-4, page 344
1) Why is human exposure to ultraviolet radiation potentially more harmful than exposure to infrared radiation?
Because infrared radiation is essentially heat energy, exposure to it is not as harmful as exposure to ultraviolet light, which is the most energetic form of sun radiation. Ultraviolet light can cause sunburns and skin cancer and can even sterilize materials by killing bacteria and destroying viruses (UV-C radiation). UV-C radiation can damage living organisms due to its energy that is high enough to break covalent bonds.
2) Describe two essential roles played by visible solar radiation.
Visible solar radiation energizes electrons in some chemical bonds. For example, this provides the energy needed for photosynthesis reactions and the visible scattering of light during sunset.
3) Explain why dry, arid regions in the United States, such as New Mexico and Arizona, experience wider air-temperature fluctuations from night to day than do states with more humid conditions, such as Florida.
Since there is less water vapor, and therefore, less greenhouse gas caused by water vapor in dry and arid regions such as New Mexico and Arizona, infrared radiation is not as stored and reradiated and reflected back into the atmosphere from the entering of UV and visible light that is transformed into IR radiation in clouds, but the visible and UV radiation directly exposed to the surface of the earth (bare in mind that most never reaches earth's surface) is used to heat earth. This results in wider air-temperature fluctuations from night to day than states with more humid conditions, for not as much heat is stored in the clouds.
4) Suppose Earth had a less dense atmosphere (fewer gas molecules) than it does now:
a. How would average daytime temperatures be affected? Why?
b. How would average nighttime temperatures be affected? Why?
a. The daytime temperatures would be much cooler, because with less greenhouse gases such as CO2 and H2O (g), less infrared radiation would be stored and reradiated and reflected back into the atmosphere from the transformation of UV and visible radiation to IR radiation in clouds (since actual IR radiation from the sun is reflected back into space). Less stored heat energy would enter throughout the day.
b. Without stored heat energy in the atmosphere, nights would be very cold without the presence of the sun!
4SBS #14-20, page 361
14) Describe how atmospheric CO2 and water vapor help maintain moderate temperatures at Earth's surface.
CO2 and water vapor are both greenhouse gases, atmospheric gas molecules that effectively absorb infrared radiation-- this infrared radiation is not directly from the sun, but is the product of transformed UV and visible radiation from the sun within the clouds. Infrared radiation is essentially heat energy, that these greenhouse gases have made from trapping UV and visible light in order to absorb, reradiate, and reflect the heat energy onto earth's surface.
15) List two natural processes and two human activities that can increase the amount of
a. CO2 in the atmosphere.
b. CH4 in the atmosphere.
a.
Natural: 1. breathing; 2. bacterial decay.
Human: 1. agricultural and industrial activities; 2. burning of fossil fuels.
b.
Natural: 1. decomposition of plants; 2. decomposition of animal wastes
Human: 1. Refining fossil fuels; 2. raising of livestock.
16) What changes in the composition of the atmosphere would cause the average surface temperature of the Earth to
a. increase?
b. decrease
a. lower altitude, increase in greenhouse/atmospheric gases, higher air pressure.
b. higher altitude, decrease in greenhouse/atmospheric gases, lower air pressure.
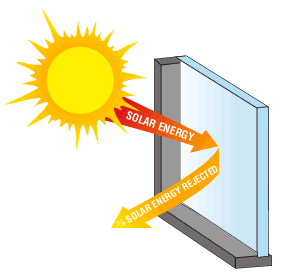
17) Explain why, on a sunny winter day, a greenhouse with transparent glass walls is much warmer than is a structure with opaque wooden walls.
What goes on in greenhouse with transparent glass imitates the way the greenhouse gases transform UV and visible radiation to IR radiation within clouds, and reradiate and reflect the heat energy within earth, or the greenhouse (since the infrared light cannot escape through the glass, like earth's CO2 and H2O gas that act as a shield). This is why on a sunny winter day, a greenhouse with transparent glass walls is much warmer than is a structure with opaque wooden walls.
18) Draw sketches to show how
a. a greenhouse works.
b. the global greenhouse effect works.
a.
b.
19) List three chemical reservoirs of carbon atoms.
Four chemical reservoirs of carbon atoms include atmospheric CO2 gas, solid calcium carbonate (CaCO3) in limestone, natural gas (methane CH4), and organic molecules.
20) Explain how, over time, a particular carbon atom can be part of the atmosphere, biosphere, lithosphere, and hydrosphere.
Carbon naturally moves within Earth's systems. The carbon atom could be part of the atmosphere as a gas as a result of photosynthesis, in the lithosphere as plant or animal waste decay, or part of the hydrosphere as limestone.
1) Why is human exposure to ultraviolet radiation potentially more harmful than exposure to infrared radiation?
Because infrared radiation is essentially heat energy, exposure to it is not as harmful as exposure to ultraviolet light, which is the most energetic form of sun radiation. Ultraviolet light can cause sunburns and skin cancer and can even sterilize materials by killing bacteria and destroying viruses (UV-C radiation). UV-C radiation can damage living organisms due to its energy that is high enough to break covalent bonds.
2) Describe two essential roles played by visible solar radiation.
Visible solar radiation energizes electrons in some chemical bonds. For example, this provides the energy needed for photosynthesis reactions and the visible scattering of light during sunset.
3) Explain why dry, arid regions in the United States, such as New Mexico and Arizona, experience wider air-temperature fluctuations from night to day than do states with more humid conditions, such as Florida.
Since there is less water vapor, and therefore, less greenhouse gas caused by water vapor in dry and arid regions such as New Mexico and Arizona, infrared radiation is not as stored and reradiated and reflected back into the atmosphere from the entering of UV and visible light that is transformed into IR radiation in clouds, but the visible and UV radiation directly exposed to the surface of the earth (bare in mind that most never reaches earth's surface) is used to heat earth. This results in wider air-temperature fluctuations from night to day than states with more humid conditions, for not as much heat is stored in the clouds.
4) Suppose Earth had a less dense atmosphere (fewer gas molecules) than it does now:
a. How would average daytime temperatures be affected? Why?
b. How would average nighttime temperatures be affected? Why?
a. The daytime temperatures would be much cooler, because with less greenhouse gases such as CO2 and H2O (g), less infrared radiation would be stored and reradiated and reflected back into the atmosphere from the transformation of UV and visible radiation to IR radiation in clouds (since actual IR radiation from the sun is reflected back into space). Less stored heat energy would enter throughout the day.
b. Without stored heat energy in the atmosphere, nights would be very cold without the presence of the sun!
4SBS #14-20, page 361
14) Describe how atmospheric CO2 and water vapor help maintain moderate temperatures at Earth's surface.
CO2 and water vapor are both greenhouse gases, atmospheric gas molecules that effectively absorb infrared radiation-- this infrared radiation is not directly from the sun, but is the product of transformed UV and visible radiation from the sun within the clouds. Infrared radiation is essentially heat energy, that these greenhouse gases have made from trapping UV and visible light in order to absorb, reradiate, and reflect the heat energy onto earth's surface.
15) List two natural processes and two human activities that can increase the amount of
a. CO2 in the atmosphere.
b. CH4 in the atmosphere.
a.
Natural: 1. breathing; 2. bacterial decay.
Human: 1. agricultural and industrial activities; 2. burning of fossil fuels.
b.
Natural: 1. decomposition of plants; 2. decomposition of animal wastes
Human: 1. Refining fossil fuels; 2. raising of livestock.
16) What changes in the composition of the atmosphere would cause the average surface temperature of the Earth to
a. increase?
b. decrease
a. lower altitude, increase in greenhouse/atmospheric gases, higher air pressure.
b. higher altitude, decrease in greenhouse/atmospheric gases, lower air pressure.
17) Explain why, on a sunny winter day, a greenhouse with transparent glass walls is much warmer than is a structure with opaque wooden walls.
What goes on in greenhouse with transparent glass imitates the way the greenhouse gases transform UV and visible radiation to IR radiation within clouds, and reradiate and reflect the heat energy within earth, or the greenhouse (since the infrared light cannot escape through the glass, like earth's CO2 and H2O gas that act as a shield). This is why on a sunny winter day, a greenhouse with transparent glass walls is much warmer than is a structure with opaque wooden walls.
18) Draw sketches to show how
a. a greenhouse works.
b. the global greenhouse effect works.
a.
b.
19) List three chemical reservoirs of carbon atoms.
Four chemical reservoirs of carbon atoms include atmospheric CO2 gas, solid calcium carbonate (CaCO3) in limestone, natural gas (methane CH4), and organic molecules.
20) Explain how, over time, a particular carbon atom can be part of the atmosphere, biosphere, lithosphere, and hydrosphere.
Carbon naturally moves within Earth's systems. The carbon atom could be part of the atmosphere as a gas as a result of photosynthesis, in the lithosphere as plant or animal waste decay, or part of the hydrosphere as limestone.
4SBS, #1-8, page 360
1) Write an equation or a sentence that describes the relationship between the frequency of electromagnetic radiation and its energy.
The frequency of electromagnetic radiation/photons and energy are directly proportional, where as wavelength of electromagnetic radiation/photons and energy are inversely proportional.
2) Why is the word spectrum a good descriptor of the types of energy found in electromagnetic radiation?
Spectrum is a good descriptor of the types of energy found in electromagnetic radiation because the spectrum shows the range of energy from high (left) to low (right).
3) Why is visible light useful in plant photosynthesis, while other forms of electromagnetic radiation are not?
Visible light is useful in plant photosynthesis while other forms of electromagnetic radiation are not because visible radiation can energize electrons in some chemical bonds-- providing energy needed for photosynthesis reactions.
4)
a. List the main types of electromagnetic radiation in order of increasing energy.
b. Describe how each radiation listed in your answer to Question 4a affects living things.
a. Infrared, visible, ultraviolet
b. Infrared radiation is essentially heat energy, which warms living things. Visible radiation can energize electrons in chemical bonds, an example being photosynthesis. Although ultraviolet radiation can cause sunburns, skin cancer, and can kill bacteria and destroy viruses, it is necessary for humans in some amounts, for exposure to it results in the production of vitamin D.
5) Ultraviolet light is often used to sterilize chemistry laboratory protective goggles. Why is ultraviolet light effective for this use, while visible light is not effective?
UV-C photons have enough energy to break covalent bonds, and therefore, lead to chemical changes in the materials exposed to radiation; UV-C radiation can kill bacteria and destroy viruses, as it can damage other living organisms.
6) Compare infared, visible, and ultraviolet radiation in terms of how well they are absorbed by the atmosphere.
Two natural major greenhouse gases, carbon dioxide and water vapor, absorb infrared radiation and reradiate the energy back to earth. On a clear day, 90% of visible radiation directed towards earth travels to Earth's surface. UV-C radiation is absorbed in the stratosphere before reaching earth's surface and most UV-A and UV-B radiation us absorbed by the stratospheric ozone layer (and does not reach the earth's surface).
7) Describe the two main effects of the solar radiation that reaches Earth's surface.
When more greenhouse gases are produced, such as carbon dioxide and water vapor, more infrared radiation is absorbed. This results in more energy reradiated on earth's surface, hotter water affecting the hydrologic cycle (more water vapor produced), and an overall hotter earth.
8)
a. Compare lake water and asphalt in terms of how readily each warms up when exposed to sunlight.
b. What properties of these two materials account for the differences in their behavior?
a. An asphalt, such as snow, sand, or concrete, warms up more quickly when exposed to sunlight. This is because when solar radiation strikes these materials, it is reflected and illuminated back into space. On the other hand, lake water does reflect light, but also stores absorbs and stores heat.
b. Water, in forms like water vapor, act as greenhouse gases and absorb infrared light and reradiate it back to earth's surface. Asphalts directly reflect light sources back into space.
Subscribe to:
Comments (Atom)